Manufacturing Process Validation Training
in Technical TrainingsAbout this training
Manufacturing Process Validation Training |TheSkope
About This Training
The Manufacturing Process Validation Training is designed to equip professionals with the knowledge and skills to validate processes effectively, ensuring product quality and compliance with industry standards. This program dives into the core principles, methodologies, and documentation practices of process validation, providing a practical approach to achieving manufacturing excellence.
Why Choose Our Manufacturing Process Validation Course?
Our course offers a complete guide to mastering process validation, tailored for manufacturing environments. It provides actionable insights and techniques that can be implemented immediately to enhance product reliability and regulatory compliance.
This training is an essential step for professionals aiming to establish robust processes and improve manufacturing outcomes.
Course Highlights
Detailed Modules: Each module covers a specific aspect of process validation, including planning, execution, and maintaining validated processes.
Experienced Trainers: Learn from experts with extensive experience in manufacturing and regulatory standards.
Hands-On Approach: Participate in exercises, simulations, and real-world case studies to reinforce your learning.
Flexible Learning Options: Gain 24/7 access to course materials, enabling you to learn at your own pace.
Detailed Modules: Each module covers a specific aspect of process validation, including planning, execution, and maintaining validated processes.
Experienced Trainers: Learn from experts with extensive experience in manufacturing and regulatory standards.
Hands-On Approach: Participate in exercises, simulations, and real-world case studies to reinforce your learning.
Flexible Learning Options: Gain 24/7 access to course materials, enabling you to learn at your own pace.
Who Should Enroll?
Quality Assurance and Quality Control Professionals
Process Engineers and Validation Specialists
Production Supervisors
Regulatory Affairs Personnel
Manufacturing Managers
Anyone involved in process validation and regulatory compliance
Quality Assurance and Quality Control Professionals
Process Engineers and Validation Specialists
Production Supervisors
Regulatory Affairs Personnel
Manufacturing Managers
Anyone involved in process validation and regulatory compliance
Why You Should Learn Manufacturing Process Validation?
Enhanced Product Quality: Validate processes to consistently meet customer and regulatory requirements.
Regulatory Compliance: Gain knowledge to comply with standards like ISO 9001, FDA, and GMP.
Risk Mitigation: Identify and eliminate process variability, reducing the risk of non-conformance.
Efficiency and Cost Savings: Optimize processes to reduce waste and improve productivity.
Career Advancement: Acquire a specialized skill set that is highly valued in manufacturing industries.
Enhanced Product Quality: Validate processes to consistently meet customer and regulatory requirements.
Regulatory Compliance: Gain knowledge to comply with standards like ISO 9001, FDA, and GMP.
Risk Mitigation: Identify and eliminate process variability, reducing the risk of non-conformance.
Efficiency and Cost Savings: Optimize processes to reduce waste and improve productivity.
Career Advancement: Acquire a specialized skill set that is highly valued in manufacturing industries.
Course Structure
Introduction to Process Validation: Learn the fundamentals of process validation, including definitions, types, and importance.
Regulatory Requirements: Understand the key regulatory guidelines and standards for process validation.
Validation Planning: Explore how to develop a comprehensive validation master plan (VMP).
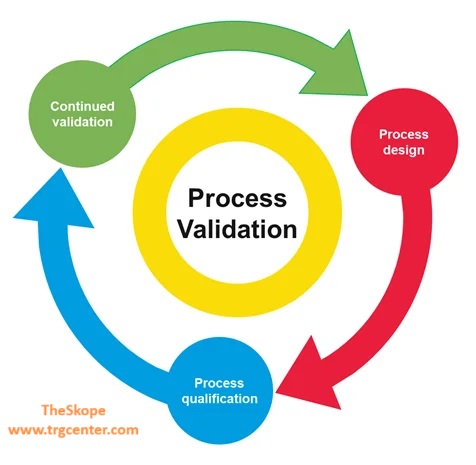
Process Qualification Stages: Delve into Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ).
Statistical Tools for Validation: Learn to use statistical methods for process control and validation.
Validation Protocols and Documentation: Understand the structure and requirements of validation protocols and reports.
Maintaining Validated Processes: Explore strategies for revalidation and monitoring validated processes.
Case Studies and Real-World Examples: Analyze successful implementations of process validation in manufacturing.
Introduction to Process Validation: Learn the fundamentals of process validation, including definitions, types, and importance.
Regulatory Requirements: Understand the key regulatory guidelines and standards for process validation.
Validation Planning: Explore how to develop a comprehensive validation master plan (VMP).
Process Qualification Stages: Delve into Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ).
Statistical Tools for Validation: Learn to use statistical methods for process control and validation.
Validation Protocols and Documentation: Understand the structure and requirements of validation protocols and reports.
Maintaining Validated Processes: Explore strategies for revalidation and monitoring validated processes.
Case Studies and Real-World Examples: Analyze successful implementations of process validation in manufacturing.
Real-World Applications
In-depth analysis of validation case studies across various manufacturing sectors.
Interactive assignments designed to simulate real-life challenges and provide practical problem-solving experience.
In-depth analysis of validation case studies across various manufacturing sectors.
Interactive assignments designed to simulate real-life challenges and provide practical problem-solving experience.
Certification Exam
Participants must complete an online assessment featuring multiple-choice questions. A passing score of 60% is required to earn certification.
Certificates are available in digital format, with an optional hard copy for Rs. 100.
Testimonials
“This training provided me with a thorough understanding of process validation. The practical approach and expert guidance were invaluable.”
— Megha Desai, Validation Specialist ⭐⭐⭐⭐⭐
“An excellent course for anyone in manufacturing. The hands-on exercises and real-world examples were incredibly helpful.”
— Vivek Joshi, Quality Manager ⭐⭐⭐⭐⭐
Enroll Today
Take the next step toward mastering process validation in manufacturing. Sign up today and ensure your processes deliver quality and compliance every time!
Comments (0)
Quiz & Certificates